EAE Induction by Active Immunization in C57BL/6 Mice
Recommended protocol for use with:
- Hooke Kit™ MOG35-55/CFA Emulsion PTX (cat. no. EK-2110), or,
- Hooke Kit™ MOG1-125/CFA Emulsion PTX (cat. no. EK-2160)
Contents
- Introduction
- Method overview
- Control of EAE severity by selection of PTX dosage
- Detailed protocol
- Expected results
- Troubleshooting
- References
- Appendix A – Mouse EAE scoring guide
- Appendix B – EAE background biology
- Appendix C – Kit selection
- Appendix D – PTX lot potency for EAE induction in C57BL/6 mice
1 Introduction
These kits are recommended for study of experimental autoimmune encephalomyelitis (EAE), including testing efficacy of potential therapeutics.
Each kit is sufficient to induce EAE in 10 mice, and consists of:
- Antigen (MOG35-55 or MOG1-125) in emulsion with complete Freund's adjuvant (three pre-filled syringes)
- Pertussis toxin (PTX) in glycerol buffer (one vial)
EAE is induced in C57BL/6 mice1 by immunization with an emulsion of MOG35-55 or MOG1-125 in complete Freund's adjuvant (CFA), followed by administration of pertussis toxin in PBS, first on the day of immunization and then again the following day.
For most studies, we recommend groups of 10 to 12 mice, with 4 to 6 mice per cage.
Eighty (80) to 100% of immunized mice will develop initial signs of EAE within 22 days of immunization – most much sooner. In a typical group of 12 untreated mice, the first mouse will experience EAE onset 9 to 14 days after immunization, with most other mice developing EAE over the following 6 to 8 days. The peak of disease is usually 3 to 5 days after onset for each mouse. The peak lasts 1 to 3 days, with scores between 2.5 and 3.5 for the 80 to 90% of mice that develop EAE.
Up to 25% of mice that develop EAE will experience partial recovery followed by an increase in severity (relapse). Relapse usually starts 24 to 28 days after immunization.
Mice are typically observed for 4 weeks, during which mice will remain chronically paralyzed.
Table 1 below gives the materials needed to induce EAE.
| Qty | Description |
|---|---|
| 1 | Hooke Kit™ MOG35-55/CFA Emulsion PTX (EK-2110) or Hooke Kit™ MOG1-125/CFA Emulsion PTX (EK-2160) (See "Appendix C - Kit selection" below.) |
| 10 | C57BL/6 mice1, females, 9 to 13 weeks old (Taconic Biosciences model B6-F or The Jackson Laboratory strain C57BL/6J) |
| 50 mL sterile polypropylene tubes 1 mL syringes 27G 1/2" (12 mm) needles |
|
| Sterile phosphate buffered saline (PBS) (standard formulation, pH 7.4, calcium-free, magnesium-free) |
1 EK-2110 (MOG35-55/CFA Emulsion PTX) can be used to induce EAE in (C57BL/6 x SJL)F1 mice. Use 50 to 75% of the C57BL/6 PTX dose for EAE in these mice.
2 Method overview
Use female C57BL/6 mice, 9 to 13 weeks old. Minimize mouse stress during all procedures (see "Troubleshooting" below).
- Acclimate mice for at least 7 days prior to immunization
- Day 0 – Select PTX dosage
- Day 0 – Inject antigen emulsion, s.c.
- Day 0 – Wait approximately 2 hours (not critical; 1 to 6 hours is acceptable)
- Day 0 – Prepare PTX solution
- Day 0 – First injection of PTX solution, i.p.
- Day 1 – Prepare PTX solution (again)
- Day 1 – Second injection of PTX solution, i.p.
Hooke recommends administration of two doses of PTX, one on the day the emulsion is administered (Day 0) and the second the following day (Day 1).
Some labs use a different PTX administration schedule; our limited experience is that PTX dosing any time between Day 0 and Day 3 results in similar EAE. However, all of Hooke’s testing is performed with dosing on Day 0 and Day 1, and this is the recommended schedule.
Most investigators score mice daily between Days 7 and 28 after immunization. (See "Appendix A – Mouse EAE scoring guide".)
The following sections provide detailed procedures.
3 Control of EAE severity by selection of PTX dosage
Many factors influence EAE severity, as summarized in Table 2 below.
| Factor | Effect |
|---|---|
| Stress before EAE onset | Strongly reduces disease severity |
| Older age | More severe disease and more uniform EAE onset |
| Gender | Female mice show more consistent disease |
| Substrain and breeder | Varies |
| PTX dose and potency | Higher doses tend to increase EAE severity |
PTX dosage is used to control EAE severity, compensating for these factors. Higher PTX doses generally increase EAE severity, lower doses reduce it (within some limits).
Because the factors above vary from lab to lab, investigators in different labs, and different experiments, require different PTX doses to induce similar EAE severity, even with identical PTX and mice.
To achieve reliable and uniform EAE development, use female mice of age 9 to 13 weeks, minimize mouse stress, and carefully select PTX dosage.
In addition, PTX potency for EAE induction can differ dramatically (up to 10 fold) between different PTX lots.
We strongly recommend kit users establish a “baseline” PTX dosage for their lab in a pilot experiment, inducing EAE in 10 mice with Hooke Kit™ MOG35-55/CFA Emulsion PTX (cat. no. EK-2110)2. See "Appendix D – PTX lot potency for EAE induction in C57BL/6 mice" for guidance.
Once a baseline PTX dose has been established, it should be adjusted for each experiment as described in the following sections.
2 Hooke Kits™ EK-2110 (MOG35-55) and EK-2160 (MOG1-125) will produce similar EAE severity with the same PTX dose.
3.1 Effect of stress
Mouse stress prior to EAE development strongly reduces EAE severity (see "Appendix B – EAE background biology"), so minimizing mouse stress is very important for successful EAE induction.
Local lab conditions such as housing, diet, temperature, humidity, noise, vibration, and pathogens, can create mouse stress and therefore affect EAE development.
Rough handling will cause mouse stress, as can moving mice on carts. Always handle mice gently, and try to perform all procedures in the mouse room.
3.2 Adjustment for treatment administration stress (prophylactic treatment only)
If mice will be treated therapeutically (starting after initial signs of EAE in each mouse), treatment has little effect on EAE development.
But if mice will be treated prophylactically (prior to EAE onset), additional PTX may be needed to compensate for the stress of treatment, according to the route and frequency of administration.
Oral (p.o.) and subcutaneous (s.c.) dosing by a skilled technician induces similar stress, while intraperitoneal (i.p.) dosing induces more stress.
For p.o. or s.c. prophylactic treatment (starting from or before immunization), increase the baseline PTX dose by 10% if mice will be dosed QD, and by 20% if dosing BID, or i.p.
Poorly tolerated vehicles or treatment compounds can be particularly stressful, as can unskilled dosing.
4 Detailed protocol
Keep all kit components cold (2 to 8 C) until use.
For the most uniform EAE development, use female C57BL/6 mice, 9 to 13 weeks old at immunization. All mice should be the same age. Male mice can be used, but will develop less consistent EAE than females.
Because EAE susceptibility is influenced by mouse stress, the same person should dose all groups in an experiment, to control for differences in injection technique.
If some mice will be treated with test compounds, negative control groups should be dosed with the same vehicle, at the same frequency, by the same route, to control for stress of treatment administration.
Acclimate mice to your lab for at least 7 days prior to immunization.
4.1 Anesthesia
Anesthesia is not required, but immunization under isoflurane anesthesia makes injection easier for those with limited experience restraining mice.
If immunizing under anesthesia, we recommend the mouse restraint and handling procedures given in Hooke protocol EAE Induction by Active Immunization in SJL Mice.
That reference is for mouse handling only; see the sections below for number and volume of injections.
4.2 Number of injections
For immunization with MOG35-55 (kit EK-2110), we recommend injection at 2 sites (0.1 mL each) as described in the next section.
For immunization with MOG1-125 (kit EK-2160), injection at 4 sites (0.05 mL each) usually induces somewhat more severe EAE than injection at 2 sites. If making 4 injections, inject mice at left and right shoulders and left and right hips.

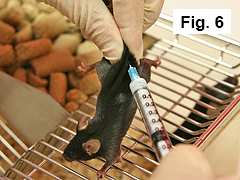
4.3 Administration of antigen emulsion
Emulsion will be administered subcutaneously, at two sites, 0.1 mL/site (0.2 mL/mouse total).
Good subcutaneous injection technique is important for successful EAE induction, both for proper emulsion administration and also to minimize mouse stress. Because EAE susceptibility is influenced by mouse stress, the same person should inject all groups in an experiment.
Figures 2 to 6 below show restraint and injection techniques for non-anesthetized mice.
(These procedures are drafted for right-handed injection; reverse if injecting with the left hand.)
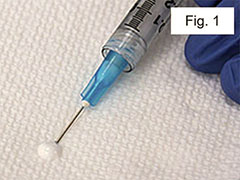
Prime the needle by pushing a small amount of emulsion out onto a sterile tissue and wiping it off (Figure 1).
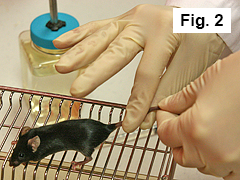
Grip mouse tail with finger and thumb of right hand (Figure 2).
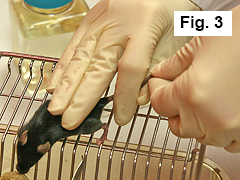
Restrain mouse against cage with three remaining fingers of right hand (Figure 3).
With left hand, restrain mouse with two fingers behind head (Figure 4).
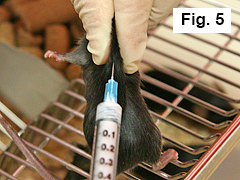
Using right hand, inject mouse subcutaneously on midline of upper back ~ 1 cm caudal of the neckline* with 0.1 mL of emulsion (Figure 5).
Keep the needle inserted into the subcutaneous space for 10 to 15 seconds to avoid leakage of the emulsion. Alternatively, a light pull on the syringe plunger will prevent leakage.
*Note: See also "Number of injections" above. If mice will be scruffed for frequent dosing (esp. via oral gavage BID or more often), inject ~ 3 cm caudal from the neckline (toward tail). While this is somewhat more difficult than injecting ~ 1 cm caudal of the neckline, it will help prevent gripping the emulsion bump during dosing, which should be avoided (see "Emulsion movement", below).
Using a similar technique, inject mouse subcutaneously on midline of lower back with 0.1 mL of emulsion (Figure 6).
Again, keep the needle inserted into the subcutaneous space for 10 to 15 seconds or lightly pull on the syringe to prevent leakage.
Repeat (2 to 6) for all mice.
Wait approximately 2 hours before proceeding with first PTX administration.
(Exact timing is not critical; 1 to 6 hours delay is acceptable, but the delay should be the same for all groups.)
4.4 Preparation of pertussis toxin solution
Each kit comes with a vial of PTX in glycerol buffer (100 to 200 ng/µL). This PTX must be diluted with cold PBS (2 to 8 C) for dosing.
PTX solution should be prepared fresh on Day 0, and again fresh on Day 1, under sterile conditions using a biosafety cabinet.
Important: Because PTX dosage affects EAE severity, pool each preparation of PTX solution for all mice in the experiment in a single 50 mL tube as follows:
-
Put PBS into 50 mL tube: Use 0.12 mL PBS for each mouse (i.e., 20% extra to allow for loss while dosing). Place the total volume for all mice into a single 50 mL sterile polypropylene tube.
Example: For 30 mice, 30 * 0.12 mL = 3.6 mL PBS into the tube.
-
Add PTX to PBS in 50 mL tube:
Briefly centrifuge the vial(s) of PTX to ensure the liquid is at the bottom of the vial(s).
-
Calculate the required amount of PTX3. Use the formula:
# of mice * PTX dose (ng) * 1.2 / PTX_concentration = µL of PTX from vial(s)
Example: 30 mice * 110 ng * 1.2 / 200 ng/µL4 = 19.8 µL
Take the calculated volume of PTX from the vial(s), and place into the tube with PBS.
Mix gently (do not vortex).
Keep PTX solution on ice until injected. Important: Complete all PTX administration within 2 hours.
3 Adjust PTX concentration to be 10x the intended dose per mL, because each mL of diluted PTX will be administered to 10 mice (0.1 mL/mouse).
4 Example only. See Appendix D for current lot concentration and potency.
4.5 Administration of PTX
Complete all PTX administration within 2 hours of preparation. (PTX in solution has a limited lifetime even when stored on ice.)
Administer PTX intraperitoneally at 0.1 mL/dose. This will be repeated 24 hours later.
Draw 1 mL PTX solution into a 1 mL syringe.
Mount a fresh 27G 1/2" (12 mm) needle.
Inject each mouse i.p. with 0.1 mL.
Repeat (1 to 3) for all mice.
22 to 26 hours later, repeat the PTX preparation and injection (steps 1 to 3 above) for all mice.
Check mice for signs of EAE daily starting on Day 7 after immunization. In most cases mice are scored until Day 28. (See "Appendix A – Mouse EAE scoring guide".)
As soon as the first signs of paralysis occur, provide mice with food pellets and wet food on the floor of the cage, and easily accessible water. HydroGel (ClearH2O, Portland ME) may be used as a source of water during the most severe paralysis.
5 Expected results
EAE will develop in 90 to 100% of untreated mice. In most mice disease onset will occur between 9 and 16 days after immunization. Most mice will reach maximum score of 3.0 to 3.5. Figure 7 illustrates typical results.
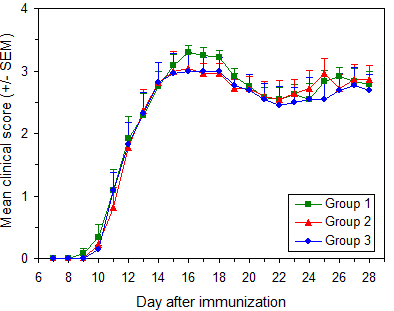
EAE induction in C57BL/6 mice
Protocol: EAE Induction by Active Immunization in C57BL/6 Mice
Data are from three independent groups in a single experiment. Immunization used Hooke Kit™ MOG35-55/CFA Emulsion PTX (EK-2110), with 11 to 13 week old female C57BL/6 mice (Taconic Biosciences).
Pertussis toxin from 3 vials was pooled before administration.
Similar results are obtained using C57BL/6 mice from The Jackson Laboratory, as well as with MOG1-125/CFA Emulsion PTX (EK-2160) using the recommended protocol.
| Group | Mice/group | Age at immunization |
Mean maximum score ± SD |
Day of onset ± SD |
Disease incidence |
|---|---|---|---|---|---|
| 1 | 11 | 11-13 weeks | 3.46 ± 0.14 | 11.3 ± 1.4 | 100 % |
| 2 | 11 | 11-13 weeks | 3.36 ± 0.32 | 11.7 ± 1.8 | 100 % |
| 3 | 11 | 11-13 weeks | 3.18 ± 0.56 | 11.3 ± 1.7 | 100 % |
5.1 Normal side effects of EAE induction
All mice will develop obvious bumps of emulsion at the injection sites 2 to 4 days after injection.
Most mice will retain these bumps for the duration of the experiment (~30 days).
Approximately 10 to 40% of mice will clear the emulsion by developing small ulcers (5 mm or less in diameter) at injection sites. These ulcers may be treated with antibiotic ointment, but this is usually not necessary – they normally heal in a few days and scars form.
The presence of small ulcers or bumps of emulsion does not correlate with EAE development.
5.2 Skin lesions
In 3 to 10% of mice deep skin lesions may develop. Smaller lesions can be treated with antibiotic ointment. For larger lesions, consult your veterinarian or euthanize the mouse.
Rarely (1-2% of cases), mice develop red, bumpy skin lesions with total hair loss (alopecia), but unbroken skin (appears similar to eczema). The affected area is typically 25 mm or more in diameter, including areas other than the place of emulsion injection. Mice will scratch the affected area. Mice with this kind of lesion will almost never develop EAE and should be euthanized and excluded from the study.
We don't know why some mice develop lesions, or a procedure to prevent them.
5.3 Emulsion movement
Some movement of the emulsion bump is expected; this typically does not impact disease. For example, when emulsion is injected approximately in the midline of the back, emulsion bumps will often appear on one side of the back. This should not be a concern unless the emulsion moves toward the neck.
Neck and head swelling can result from grasping the emulsion injection site during dosing, which can move the emulsion toward the neck. Such swelling often requires euthanasia.
While rare, this side effect is more common when mice are dosed frequently (more than once daily) via oral gavage or intraperitoneal dosing. Emulsion movement is less likely if mice are administered emulsion caudal to the place mice will be gripped during dosing. Placing the rostral injection ~ 3 cm caudal of the neckline is usually sufficient to prevent this problem.
6 Troubleshooting
Successful induction of EAE requires low-stress mouse handling and husbandry procedures, good injection technique, use of appropriate mice, and good quality, stable, antigen emulsion together with the correct amount of pertussis toxin.
Test articles that cause local irritation or tissue damage (usually when administered i.p. or s.c.) will reduce or prevent development of EAE. Such formulations cannot be successfully tested in EAE unless a better tolerated formulation is developed.
Minimizing mouse stress is very important for successful EAE induction:
- Acclimatize mice to your lab for at least 7 days before immunization.
- House mice in a quiet environment, without excessive noise or vibration.
- Avoid excessive handling of mice. Handle mice gently.
- Try to do all procedures in the mouse room. Avoid moving mice on carts.
- Follow recommended injection procedure (see "Administration of antigen emulsion" above).
If you have difficulty inducing EAE:
- Verify EAE develops in untreated mice.
- Use female C57BL/6 mice, 9 to 13 weeks old at immunization.
- Use only isoflurane anesthesia (anesthesia is not necessary).
- Administer all PTX within 2 hours of preparation; keep on ice.
- Adjust dose of PTX (see "Control of EAE severity by selection of PTX dosage") above.
If neck or head swelling occurs, consult a veterinarian; euthanasia may be necessary:
- Try to avoid gripping emulsion injection areas to prevent emulsion movement towards neck and shoulders/limbs.
- If frequent gripping is required for dosing, instead of placing rostral injection between shoulder blades, inject in midback.
Contact with any questions.
7 References
[1] Lyons JA et al, Eur J Immunol 29:3432 (1999)
[2] Mendel I et al, Eur J Immunol 25:1951 (1995)
[3] Marusic S et al, J Exp Med 202:841 (2005)
[4] Thakker P et al, J Immunol 178:2589 (2007)
Appendix A - Mouse EAE Scoring Guide
Typically, EAE is scored on scale 0 to 5. Most researchers also give mice "in-between" scores (i.e. 0.5, 1.5, 2.5, 3.5) when the clinical picture lies between two defined scores.
The scoring method differs slightly depending on the stage of disease (onset/peak vs. recovery), for each individual mouse.
Reliable EAE scoring requires skill which comes after considerable experience. To avoid unconscious bias in scoring, we strongly recommend that mice should be scored blind, by a person unaware of which mice have received which treatment.
We recommend the following scoring guidelines for mice during onset and peak of EAE:
Mouse EAE scoring – onset and peak
| Score | Clinical observations |
|---|---|
| 0.0 | No obvious changes in motor function compared to non-immunized mice. When picked up by base of tail, the tail has tension and is erect. Hind legs are usually spread apart. When the mouse is walking, there is no gait or head tilting. |
| 0.5 | Tip of tail is limp. When picked up by base of tail, the tail has tension except for the tip. Muscle straining is felt in the tail, while the tail continues to move. |
| 1.0 | Limp tail. When picked up by base of tail, instead of being erect, the whole tail drapes over finger. Hind legs are usually spread apart. No signs of tail movement are observed. |
| 1.5 | Limp tail and hind leg inhibition. When picked up by base of tail, the whole tail drapes over finger. When the mouse is dropped on a wire rack, at least one hind leg falls through consistently. Walking is very slightly wobbly. |
| 2.0 | Limp tail and weakness of hind legs. When picked up by base of tail, the legs are not spread apart, but held closer together. When the mouse is observed walking, it has a clearly apparent wobbly walk. One foot may have toes dragging, but the other leg has no apparent inhibitions of movement. - OR - Mouse appears to be at score 0.0, but there are obvious signs of head tilting when the walk is observed. The balance is poor. |
| 2.5 | Limp tail and dragging of hind legs. Both hind legs have some movement, but both are dragging at the feet (mouse trips on hind feet). - OR - No movement in one leg/completely dragging one leg, but movement in the other leg. - OR - EAE severity appears mild when picked up (as score 0.0-1.5), but there is a strong head tilt that causes the mouse to occasionally fall over. |
| 3.0 | Limp tail and complete paralysis of hind legs (most common). - OR - Limp tail and almost complete paralysis of hind legs. One or both hind legs are able to paddle, but neither hind leg is able to move forward of the hind hip. - OR - Limp tail with paralysis of one front and one hind leg. - OR - ALL of:
|
| 3.5 | Limp tail and complete paralysis of hind legs. In addition to: Mouse is moving around the cage, but when placed on its side, is unable to right itself. Hind legs are together on one side of body. - OR - Mouse is moving around the cage, but the hind quarters are flat like a pancake, giving the appearance of a hump in the front quarters of the mouse. |
| 4.0 | Limp tail, complete hind leg and partial front leg paralysis. Mouse is minimally moving around the cage but appears alert and feeding. Often euthanasia is recommended after the mouse scores 4.0 for 2 days. However, with daily s.c. fluids most C57BL/6 mice may recover to 3.5 or 3.0, while SJL mice may fully recover even if they reach score 4.0 at the peak of disease. When the mouse is euthanized because of severe paralysis, a score of 5.0 is entered for that mouse for the rest of the experiment. |
| 4.5 | Complete hind and partial front leg paralysis, no movement around the cage. Mouse is not alert. Mouse has minimal movement in the front legs. The mouse barely responds to contact. Euthanasia is recommended. When the mouse is euthanized because of severe paralysis, a score of 5.0 is entered for that mouse for the rest of the experiment. |
| 5.0 | Mouse is spontaneously rolling in the cage (euthanasia is recommended). - OR - Mouse is found dead due to paralysis. - OR - Mouse is euthanized due to severe paralysis. |
In the recovery stage of EAE, most mice will have a tail that is no longer limp but is not normal either; it feels rigid and is "hooked". The hind legs may start moving (pedaling), but the mouse cannot walk. Either change makes scoring difficult.
We recommend the following modifications to the above scoring criteria for these mice:
Mouse EAE scoring – modified
| Score | Clinical observations |
|---|---|
| 0.0 | When held by the base of tail, tail is somewhat “hooked” and rigid, but tail makes complete rotations around the body axis (“helicopter”). Mouse is healthy. No signs of wobbling. |
| 0.5 | Mouse appears normal but tail is “hooked” and rigid. Tail does not make complete rotations around the body axis (“helicopter”). Mouse is healthy. No signs of wobbling. |
| 3.0 | Mouse is found on its side (as described for score 3.5 above), but there is excessive hind leg movement. Mouse cannot walk. - OR - Mouse has a wobbly walk (as described for score 2.5 above), and is unable to take more than two steps without falling on its side. The mouse is unable to right itself. - OR - Mouse has poor movement in the hind legs (as described for score 2.5 above), and has partial front leg paralysis evidenced by head held lower than normal and mouse's inability to right itself when placed on its side. |
| All other scores | Subtract 0.5 from the score of all mice with either a rigid, “hooked” tail or pedaling of hind legs. |
Appendix B – EAE background biology
Overview
Experimental autoimmune encephalomyelitis (EAE) is the most commonly used mouse model of human multiple sclerosis (MS). Because of its many similarities to MS, EAE is used to study pathogenesis of autoimmunity, CNS inflammation, demyelination, cell trafficking and tolerance induction.
EAE is characterized by paralysis, CNS inflammation and demyelination. EAE is mediated by myelin-specific CD4+ T cells, but CD8+ cells and B cells may also play a role in some models of EAE.
EAE is induced in C57BL/6 mice by immunization with MOG35-55 or MOG1-125 in CFA emulsion followed by administration of pertussis toxin (PTX) in PBS. The emulsion provides antigen which initiates expansion and differentiation of MOG-specific autoimmune T cells.
PTX enhances EAE development by providing additional adjuvant and facilitating entrance of autoimmune T cells into the CNS.
Fingolimod (FTY720, Gilenya) is the most commonly used positive control in this model.
Experimental model
Chronic EAE develops in C57BL/6 mice after immunization with an emulsion of MOG35-55/CFA or MOG1-125/CFA followed by injection of pertussis toxin. This model is used to test the potential of compounds to prevent or mitigate EAE disease. It can be run with the compound dosed from the time of immunization (prophylactic treatment), or with the aim of reversing the course of disease and facilitating recovery by dosing the compound from the time of EAE onset (therapeutic treatment).
The MOG1-125 antigen is used for testing therapeutics which specifically target B cells; EAE development after immunization with MOG1-125 is reported to be impaired in B cell deficient mice.
The model uses female C57BL/6 mice of age 9 to 13 weeks at the start of the study. Typically, EAE develops 8 to 18 days after immunization. EAE development is usually followed for 4 weeks (28 days) after immunization.
Stress prior to EAE development reduces EAE severity. Aside from any compound effects, the administration of treatment during the disease induction period (~0-10 days after immunization) postpones disease onset and reduces disease severity. This is due to the stress of compound administration and the effects of the vehicle on the mice. The more frequent the administration and the less tolerated the vehicle, the greater the impact on disease development.
The stress of treatment and administration of vehicle has little effect on disease development after clinical signs of EAE have appeared.
Prophylactic treatment
In prophylactic studies, treatment begins before disease onset, at the time of immunization and group assignment.
Prophylactic studies assess if treatment will affect the course of disease both before and after the first clinical signs of EAE.
To compensate for the stress of treatment in prophylactic treatment studies and achieve the target disease severity, we induce EAE with a higher dose of pertussis toxin than used in therapeutic studies. The dose of pertussis toxin is based on our prior experience with the model, according to the expected stress due to dosing (route, frequency, and formulation of vehicle).
Mice are assigned to treatment groups in a balanced manner to achieve groups with similar distributions of body weights.
In prophylactic studies, median time to disease onset is usually the most sensitive measure of compound efficacy.
Small changes in the immune response can result in postponed disease onset - suppression of T cell activation and proliferation, antigen presentation, differentiation into Th1 and/or Th17 cells will all result in postponed onset of EAE.
Delayed onset of EAE accompanied with lower maximum severity indicates overall efficacy of treatment compared to the negative control group.
Some studies will show postponed EAE onset without other significant compound effects. In these cases the compound may affect an early pathway in immune response development, but eventually redundant processes compensate for the loss of the blocked pathway. Another possible explanation is that the drug was not able to maintain the blockade of the pathway for the duration of the study.
Compounds that delay EAE onset often also cause higher end EAE scores than in the vehicle-treated mice. Usually this is not caused by the compound making EAE worse but by the peak of disease in the compound-treated mice being postponed and coinciding with the period of recovery for the vehicle-treated mice.
FTY720, the most common positive control in this model, is a very potent inhibitor of EAE development when dosed p.o. at 0.5 mg/kg or more QD. When dosed at ~0.1 mg/kg, FTY720 only postpones EAE onset without reducing maximum disease severity.
When a compound is dosed prophylactically, the most important readout of efficacy is reduction in maximum disease severity (mean maximum score, MMS). Reduced MMS indicates an overall reduction in EAE severity.
Therapeutic treatment
Therapeutic treatment studies usually begin treatment at the time of EAE onset. In some studies, when compounds are tested for their ability to reverse the course of chronic EAE, treatment is initiated 7-14 days after disease onset. Mice are assigned to treatment groups as they develop EAE (rolling enrollment) or at a fixed time after immunization, but always in a balanced manner to achieve groups with similar time of EAE onset and similar EAE onset scores. If enrollment is after EAE onset, mice are also balanced for maximum score before enrollment.
To obtain highly uniform groups, EAE is usually induced in 10% more mice than needed in the study; this provides a larger pool of mice for balancing the groups.
Therapeutic studies assess if treatment will reverse the course of disease or improve recovery from EAE.
Results are usually analyzed by synchronizing scores to the day of disease onset for each mouse.
The most important readout in this model is the average end clinical EAE score. This is the clinical outcome of the experiment; a reduction compared to the negative control group indicates treatment efficacy.
Course of EAE development in untreated mice
Individual mice will have somewhat differing courses of disease. Most mice show initial signs of EAE between 9 and 14 days after immunization. Once EAE starts, the peak of disease almost always occurs 3-4 days later. The maximum score continues for several days and then mice partially recover. In some mice, disease will stay at maximum severity until the end of the study. Less often, a mouse will stay at the peak severity for only one day and then start recovering.
The extent of recovery largely depends on the maximum severity reached by the mouse. In our experience mice which reach EAE score of 2.5 never experience full recovery. This is most likely due to axonal damage which occurs in mice with extensive inflammation. Most untreated or vehicle-treated mice will not fully recover, but their end score will usually be 0.5 to 1.5 points lower than their maximum score. About 25% of untreated or vehicle-treated mice show worsening EAE between 24 and 28 days after immunization, resembling a relapse. Spinal cords of these mice at the time of EAE worsening have a large number of inflammatory foci (≥ 7 foci per section), similar to histological findings at the time of EAE onset and peak, suggesting that these are true relapses with a new wave of inflammation in the spinal cords.
When mice are followed for a longer period of time (longer than 6 weeks), disease usually slowly increases in severity, resembling the chronic progressive course of disease observed in human MS patients.
During the course of EAE, changes in body weight reflect disease severity. Mice often lose a small amount of weight on the day following immunization. This appears to be due to effects of the administered adjuvant and pertussis toxin. Mice then steadily increase their body weight until disease onset. On the day of EAE onset, mice consistently lose 1-2 g of their body weight (5-10% of body weight). The weight loss continues with the progression of EAE severity, with the loss reaching around 20% of their pre-onset body weight at the peak of disease. The weight loss is most likely due to both paralysis and reduced food intake as well as high production of pro-inflammatory cytokines such as TNF during the acute phase of inflammation. After the peak of disease is reached, mice slowly gain weight, even if their clinical score does not improve. This increase in weight may be due to down regulation of inflammation which results in lower levels of pro-inflammatory cytokines in blood. Untreated or vehicle-treated mice usually have around 90% of their pre-immunization body weight 28 days after immunization.
Histology
Typically, histological analysis is performed either at the end of the study (usually around 28 days after immunization) or at the time when the vehicle group reaches peak of disease (usually 14-18 days after immunization).
Inflammation in EAE normally starts in the lumbar region of the spinal cord, spreading to the entire spinal cord by the peak of disease.
At onset of disease the number of inflammatory foci correlates strongly with disease severity. The number of foci increases somewhat until the peak of disease, when 6-15 inflammatory foci/section are typically found throughout the spinal cord. In the chronic stage of EAE (starting several days after the peak of disease), many inflammatory foci resolve, typically resulting in 3-4 inflammatory foci in each spinal cord section by approximately 28 days after immunization.
Because the largest numbers of inflammatory foci are present early in the course of disease, if histological analysis is performed at the end of the study, mice which have late EAE onset often have more inflammatory foci in their spinal cords than might be expected from their clinical score. For example, in a 28 day study a mouse with EAE onset on 27 days after immunization and an end clinical score of 2 will likely have more inflammatory foci than a mouse with EAE onset 9 days after immunization and an end score of 3.5. Similarly, a mouse which relapses shortly before the end of the study (relapse is defined as 1 or more points of increase in clinical score) will usually have more inflammatory foci at the end of the study than a mouse with stable chronic disease, even if the two have the same clinical score at the end of the study.
Demyelination is usually not found during the first two days after disease onset, but is found at the peak of disease (4 to 5 days after EAE onset) and continues during the chronic phase of EAE. Demyelination scores do not change much between the peak and 28 days after immunization and usually average between 1.2 and 2.5.
Demyelination is scored in anti-myelin basic protein (anti-MBP, using immunohistochemistry) and hematoxylin & eosin (H&E) stained sections.
In anti-MBP sections, demyelination is observed as conspicuous unstained areas in white matter tracts, and is associated with presence of large vacuoles.
In H&E stained sections, disruption of normal structure and the presence of large vacuoles is indicative of demyelination.
Apoptotic cells are identified in H&E sections, and are usually not found during the first two days of disease development. They are found at the peak and during the chronic stage of EAE. The average number of apoptotic cells is usually between 2 and 4 per section.
Appendix C – Kit selection
Hooke Kit™ EK-2110 contains MOG35-55 as the antigen; kit EK-2160 contains MOG1-125.
Both kits are recommended for use with female C57BL/6 mice at age 9 to 13 weeks. EK-2110 (MOG35-55) may also be used with (C57BL/6 x SJL) F1 mice; with these mice use a smaller dose of PTX (typically 50 to 75% of the dose needed in C57BL/6 mice).
Either antigen can be used for study of EAE development, including efficacy testing of potential therapeutics.
The MOG1-125 antigen is recommended for testing therapeutics which specifically target B cells; EAE development after immunization with MOG1-125 is reported to be impaired in B cell deficient mice [1].
For information on choice of model and antigen, please see Hooke's Learning Center (http://hookelabs.com/learning/#eaetradeoffs).
Appendix D – PTX lot potency for EAE induction in C57BL/6 mice
PTX potency for EAE induction can differ by up to 10-fold between different PTX lots. Therefore, dosage must be adjusted when switching to a new lot of PTX (in addition to adjustments for local lab conditions and mouse stress as described above).
Unfortunately, EAE severity is not linear with PTX dose, and the shape of the dose-response curve differs with each PTX lot.
As a guide for users of earlier lots of Hooke PTX, Table D1 below shows the relative potency Hooke PTX lots, based on our in vivo experience inducing EAE.
Hooke’s recommended baseline PTX dose for each of the 2 administrations (on Days 0 and 1) is:
- For EK-2110 kits (containing MOG35-55 antigen): 85 to 110 ng (PTX lot 1016)
- For EK-2160 kits (containing MOG1-125 antigen): 110 ng (PTX lot 1014)
Note that Hooke supplies different lots of PTX with different antigens – we recommend these lots of PTX.
for inducing EAE in C57BL/6 mice
| PTX lot # | Potency (relative) | Concentration | Recommended EK-2110 dose (MOG35-55) | Recommended EK-2160 dose (MOG1-125) |
|---|---|---|---|---|
| 1001 | 0.6 | N/A, lyophilized | Lots no longer available | Lots no longer available |
| 1002 | 0.6 | N/A, lyophilized | ||
| 1003 | 0.3 | N/A, lyophilized | ||
| 1004 | 0.15 | N/A, lyophilized | ||
| 1005 | 0.3 | N/A, lyophilized | ||
| 1006 | 1.5 | 0.2 mg/mL | ||
| 1007 | 1.5 | 0.1 mg/mL | ||
| 1008 | 0.75 | 0.2 mg/mL | ||
| 1012 | 0.5 | 0.2 mg/mL | Custom order only | Not recommended |
| 1013 | 0.5 | 0.2 mg/mL | No longer available | No longer available |
| 1014 | 0.75 | 0.2 mg/mL | Not recommended | 110 ng |
| 1015 | 0.5 | 0.2 mg/mL | No longer available | No longer available |
| 1016 | 0.5 | 0.2 mg/mL | 85 to 110 ng | Not tested |
Note: If the lot of PTX supplied with your kits do not appear in Table D1 above, see hookelabs.com/protocols for an updated protocol.
We do not recommend doses greater than 150 ng each day. These doses may produce lower average severity than lower doses (~110 ng), and variability within a group increases, with some mice developing rather mild disease, while others develop excessively severe disease.
As stated, the above doses are guidelines, and should be adjusted for your specific laboratory conditions and expected mouse stress.
Version: 2025-09-12


_150px.jpg)